


How electrons 'conduct' themselves
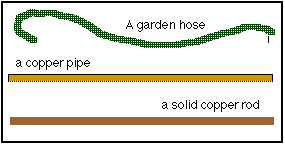
What are the differences between the three items in the figure below?
The garden hose will 'conduct' water, so will the copper pipe, but the solid one will not. By contrast, the garden hose will not conduct electricity. The copper pipe will, and the copper rod? If anything, it will do it just a wee bit better.
The other difference is that we will see the water coming out of the end of the hose or the pipe. In electricity conduction, however, WE CANNOT SEE THE ELECTRONS MOVE. We can only discover what they DO. This will become an important issue later, when we talk about safety in working with electricity.
Of course you know that metals conduct electricity very well. Insulators do not. How do we know if some object conducts or not? Simple. Everyone who is interested in electronics, even if he is only dabbling in it, can easily and cheaply build a simple instrument - a buzzer, a couple of flexible leads and a battery. This is known as a 'continuity indicator' and those of you who have built the tester in Fact Sheet No 5 have surely experimented with it. You may have found out for yourselves how useful it can be to see if some material conducts electricity or not. (With a buzzer and a Morse key, the tester can even be used to learn and practice the Morse alphabet.)
Certain metals have a somewhat lower conductance than others. Lower conductance means that they have higher resistance to the current flow. One of the best conductors is copper (it is also real fun to solder copper wires, provided we have good clean surfaces) which means that the resistance of copper is low. Aluminium conducts almost as good. In fact aluminium is commonly used in long-distance power transmission cables. Aluminium has rather low weight, another advantage when you think of miles and miles of overland power lines.
Then there are metals and alloys that have an especially high resistance/low conductance. You find those in the elements in electric heaters or your soldering iron. When you switch them on, large power is being dissipated in those wires and so they create heat and even light.
Water, perfectly clean, distilled water that is, has a very high resistance. As soon as you put impurities, salt, vinegar or fruit juice into the water, the resistance drops. If you have a multimeter, on the Ohm setting you can watch the resistance change. Put two electrodes, such as stainles steel spoons, (make sure they are made from the same metal, otherwise it becomes a battery) immerse them into the water while making sure they do not touch each other. Now connect them to the meter. You can do some experiments with water and read the change of resistance on the meter as you add different substances.
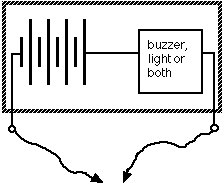
Principle of a continuity tester
Insulators, naturally, have an almost infinitely high resistance to current flow. Ceramics and many plastics are excellent insulators. One insulator is all around you at this very moment. It is air. But then, if you have those extremely high voltages from something like a Van der Graf generator *), on a humid or rainy day even the moisture in the air may conduct enough to prevent this type of generator from producing really big sparks.
* * *
Everyone knows what a switch is and what it does. You may be surprised, however, how much there is to switches that you simply didn't know. Find out in our next Fact Sheet No 10, won't you.
See you in Electronics Fact Sheet No 10.
If you have any ideas of your own that you may wish to pass on or may ask a question or two you may send them to my mail address and I will send you a reply.
Click here to return to Fact Sheet links
Go to author's home page.Copyright& © 1997-2010 Peter Schmedding, Child Development Projects, Canberra, Australia.
*) A 'Van der Graaff generator' is an experimental instrument that produces extremely high voltages while the current is very low indeed. The voltage creates some phantastic display of sparks. Generators of this type may be found in technical institutions and some electronic laboratories. Their use can be dangerous. There are interesting web pages on the net. Search for "Van_de_Graaff".